
Chemistry only
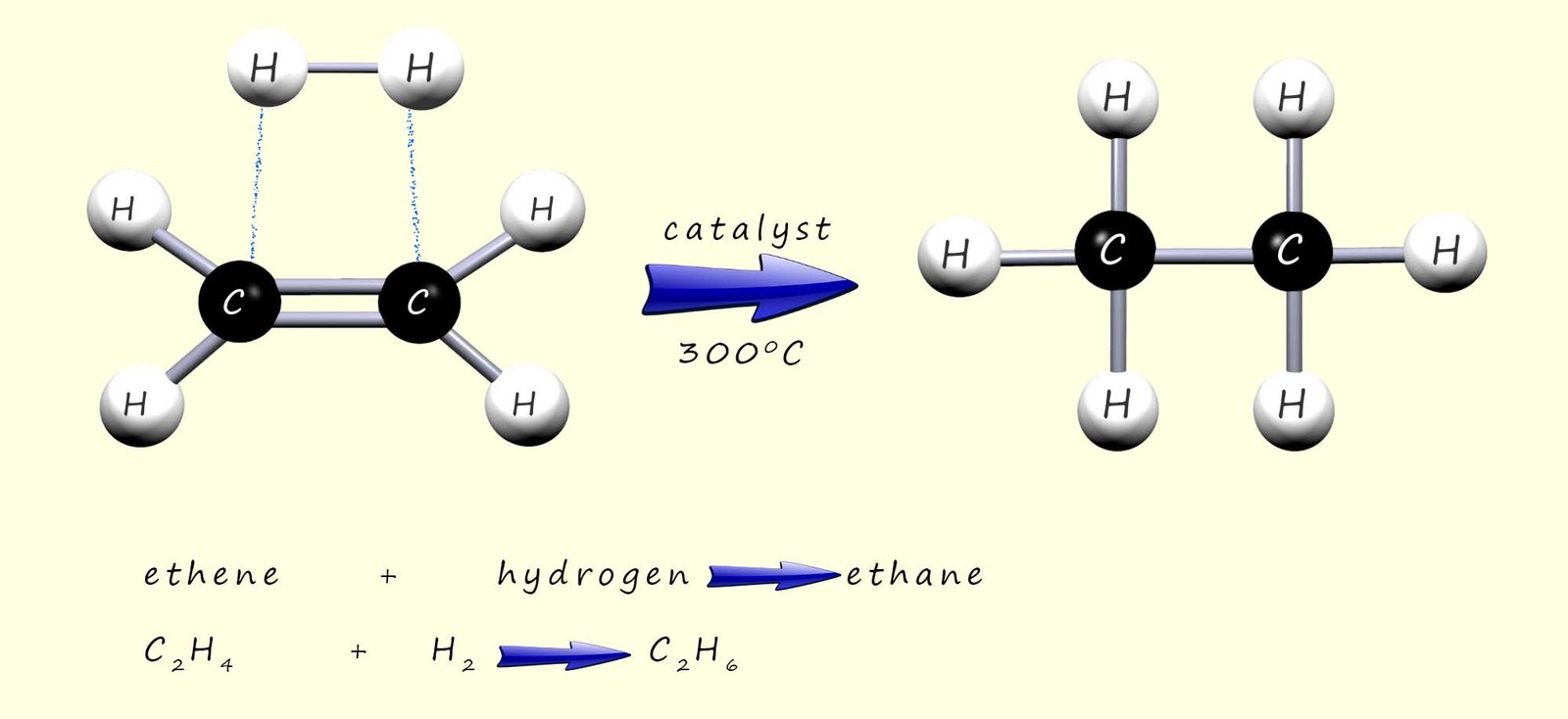
Alkenes contain a double carbon carbon covalent bond (C=C); this is the reactive functional group present in all alkene molecules. As soon as you see this functional group in any
molecule as a chemist you should think of only one thing- addition reactions! This carbon carbon double covalent bond (C=C) in an alkene
molecule is vulnerable to attack by other substances which simply add across it.


If all the carbon carbon double bonds in the vegetable oil molecule are removed by hydrogenation then the product will likely be a hard solid fat. However by hydrogenating only a specific number of the carbon carbon double bonds (C=C) present it is possible to determine just how hard and spreadable the margarine formed will be.
Other small molecules such as bromine and iodine will also add across the carbon carbon double covalent bond (C=C) to form saturated molecules. The diagram below show the products of the addition reaction of bromine to the unsaturated alkene ethene.
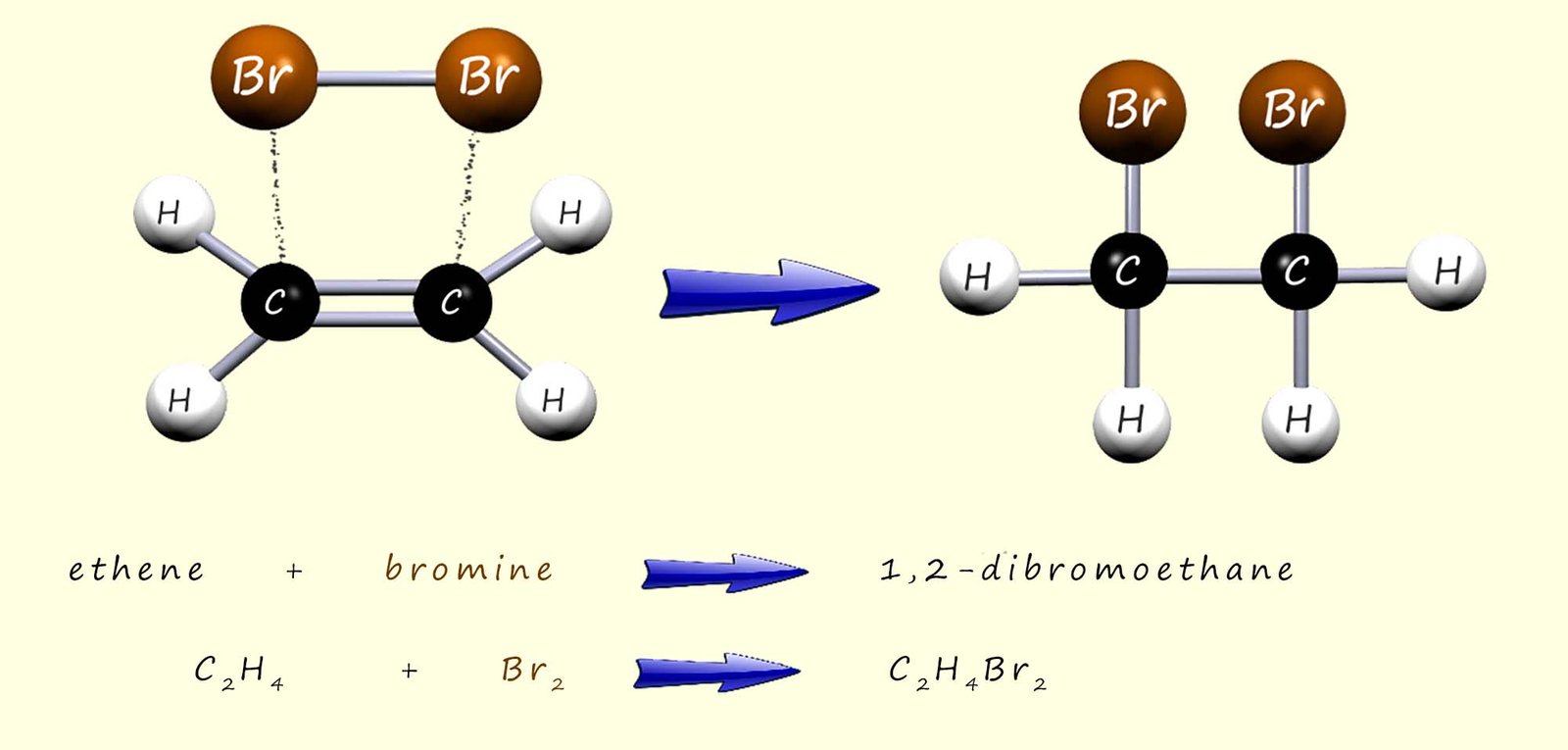
A similar addition reaction will occur if the bromine is swapped for chlorine (Cl2) or iodine (I2) and the product of the reaction this time will be 1,2-diiodoethane. Note the numbers 1,2- in the names of the compounds mentioned above are simply used to indicate which carbon atoms the bromine or iodine atoms have added to, however I am sure you would agree that the addition of a halogen (halogenation) across the carbon-carbon double bond (C=C) is very similar to the addition of hydrogen in the hydrogenation reaction above.
It is also possible to add steam across the C=C bond in alkenes to make alcohols. This method of making alcohols is called direct hydration. It requires a fairly high temperature of around 3000C and a pressure of about 65 atmospheres as well as a phosphoric acid catalyst. The reaction is similar to the examples above in that a small molecule; in this case steam adds across the C=C bond in an alkene, this is shown below using the alkene ethene as an example, addition of steam to ethene will produce the alcohol ethanol:
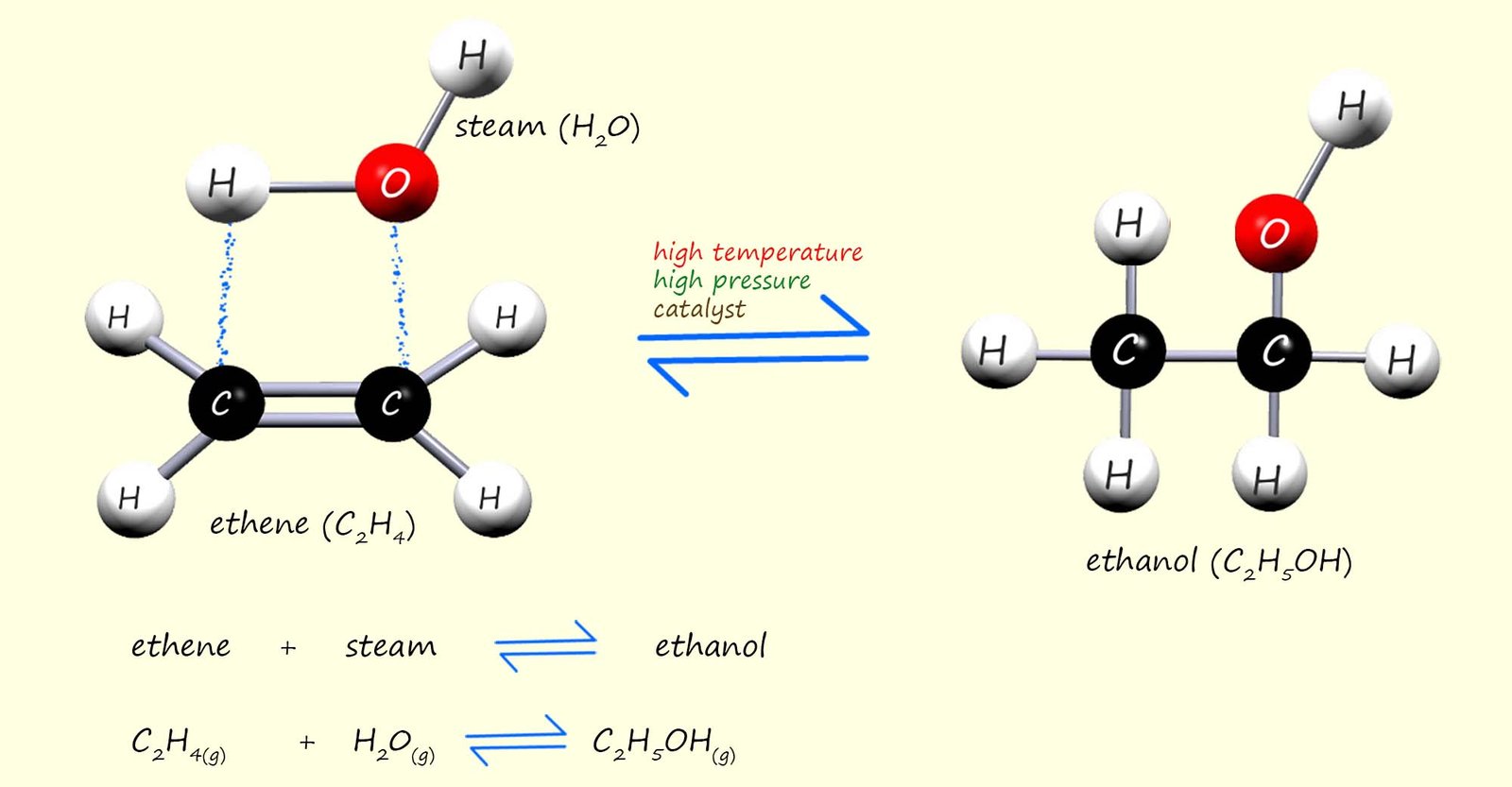
From the equation in the image above you can see that this hydration reaction is a reversible reaction. This means that the products of the reaction will be a mixture of ethanol, steam and unreacted ethene, in fact very little of the ethene and steam react when they first enter the reaction chamber. This mixture of substances formed from the hydration reaction then leaves the reaction vessel and enters a condenser, this is outlined below:
In the condenser the steam and ethanol condense and turn back into liquids while the unreacted ethene is recycled back through the reactor to react with more steam, this way the yield of the reaction can be greatly increased. The ethanol and water formed during the reaction mix freely to form a solution which leaves the condenser. The ethanol produced by direct hydration can be separated from this solution by fractional distillation.
In fact the addition of bromine across a carbon carbon double covalent bond (C=C) is used as a test for the presence of
unsaturation in a molecule.
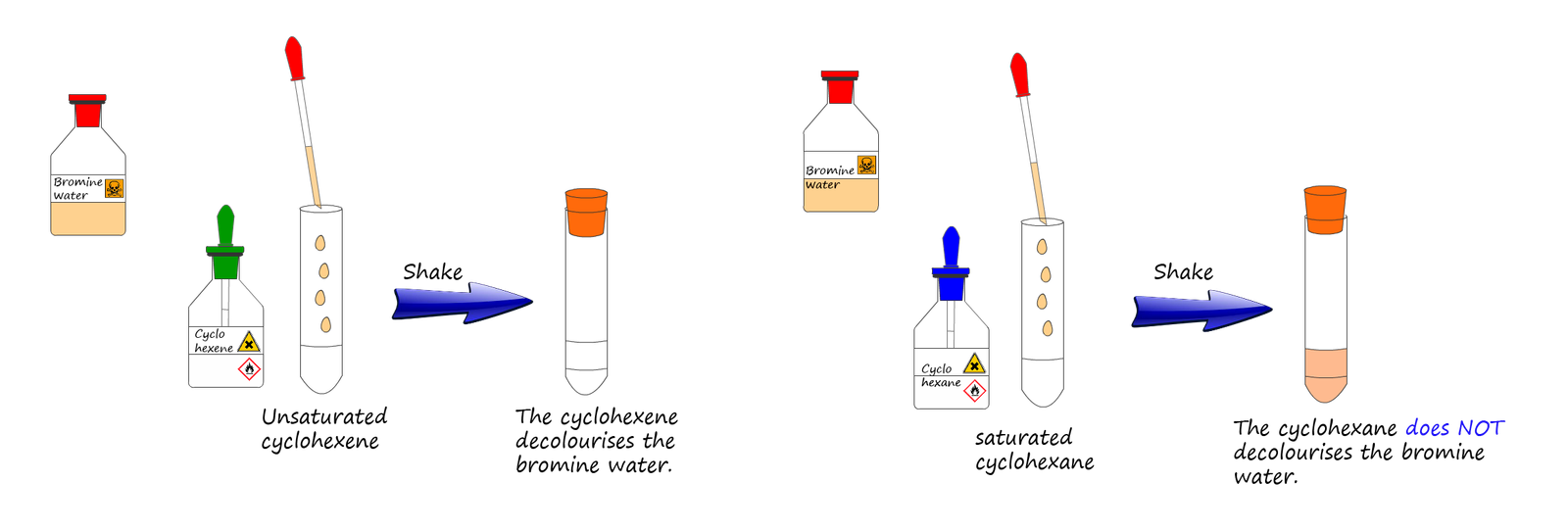
A few millilitres (ml) of liquid bromine is dissolved in water to form a red-brown solution called bromine water. When bromine water is
added to a suspected unsaturated substance
in a boiling tube and shaken then if the substance is unsaturated the bromine water will decolourise almost immediately. If
the substance in the boiling tube is saturated then the bromine water will decolourise very very slowly.
You may get to try out this test for unsaturation in the lab yourself. The two alkanes and alkenes that are most commonly used for this unsaturation testing are cyclohexane and cyclohexene. These are liquid alkanes and alkenes that are available in most schools. They are both clear liquids that like most organic substances do not mix with water but instead float on top of an aqueous (watery layer) since they are less dense than water.
The procedure to test these two substances for the presence of a C=C double bond is as follows:
This is shown in the drawing below:
Organic substances such as cyclohexane and cyclohexene are like oil they do not mix with water. Instead as shown in the image above they float on top of the water or any aqueous solution. However halogen molecules such as chlorine, bromine and iodine are more soluble in organic solvents such as cyclohexane or cyclohexene than they are in water. So if bromine is given an option of dissolving in water or in an organic solvent it will dissolve in the organic solvent.
In the boiling tube containing the bromine water and cyclohexane in the image above the two layers are shown coloured red orange due to the presence of dissolved bromine in each layer. However if the boiling tube is left to stand eventually any dissolved bromine in the aqueous layer will move into the organic cyclohexane layer simply because it is more soluble in the organic cyclohexane layer. This is outlined in the image below:
